Problem Analysis
🐻 The Brown Bear Paradox: Surviving Without Muscle Loss



❄️ What is hibernation?
Hibernation is a survival strategy that allows mammals to endure harsh winters by:
- Lowering body temperature
- Reducing metabolic rate
- Decreasing heart rate, oxygen consumption, and neural activity
- Entering prolonged fasting and inactivity
This normally causes severe muscle atrophy in non-hibernators due to an imbalance between protein synthesis and degradation.
💪 Why are brown bears special?
Brown bears (Ursus arctos) hibernate for 5–7 months:
- No food or water
- Minimal movement
- Body temperature drops to ~33.5 °C
Yet, they:
- Lose only 10–15% muscle mass
- Reduce metabolism by 20–50%
- Avoid metabolic diseases common in humans under inactivity
This makes them a biological paradox and an ideal model for studying muscle preservation.
🧪 Seasonal Changes in Bear Blood Plasma


🧬 Proteomics & metabolomics findings
Comparing summer vs winter bear plasma revealed:
- Higher total protein concentration in winter, despite lower levels of many individual proteins → Likely due to dehydration, reduced protease activity, and longer protein half-life
- Strong upregulation of lipid-related molecules:
- LDL cholesterol
- Triglycerides
- Phospholipids
- Apolipoproteins
- Serum albumin (fatty acid transport)
🛡️ Immune system shift
- Increase in innate immune components (e.g. lysozyme C, cathelicidin)
- Decrease in adaptive immune proteins (complement, antibodies) → Bears rely mainly on innate immunity during hibernation
🧠 Key Plasma Factors of Interest
🔴 Haptoglobin (HP)
- Increased 4.7-fold in winter
- Binds free hemoglobin, preventing ROS formation
- Likely functions as stress protection, not inflammation
🧪 Sex Hormone Binding Globulin (SHBG)
- Increased ~45-fold in winter
- Regulates hormone bioavailability
- In humans, low SHBG is linked to:
- Type II diabetes
- Obesity
- Cardiovascular disease
- Muscle weakness → High SHBG may help bears maintain muscle and metabolic health
🧬 Amino acid and nitrogen conservation
Winter bears show higher levels of:
- Glutamine, glutamate, lysine, histidine, proline
- Carnosine, ornithine, acetylornithine
At the same time:
- Methionine sulfoxide (oxidative stress marker) is reduced 5-fold
Despite being anuric and having reduced kidney filtration, bears:
- Recycle urea via gut bacteria → ammonia → reused nitrogen → Preserves protein and prevents muscle loss
🧫 Why Use Bear Serum Experimentally?


- Bear serum contains bioactive factors that:
- Protect human muscle cells
- Regulate TGF-β / BMP signaling
- Exact molecules are still unknown
- Goal: identify circulating anti-atrophy signals
🪱 Why C. elegans?
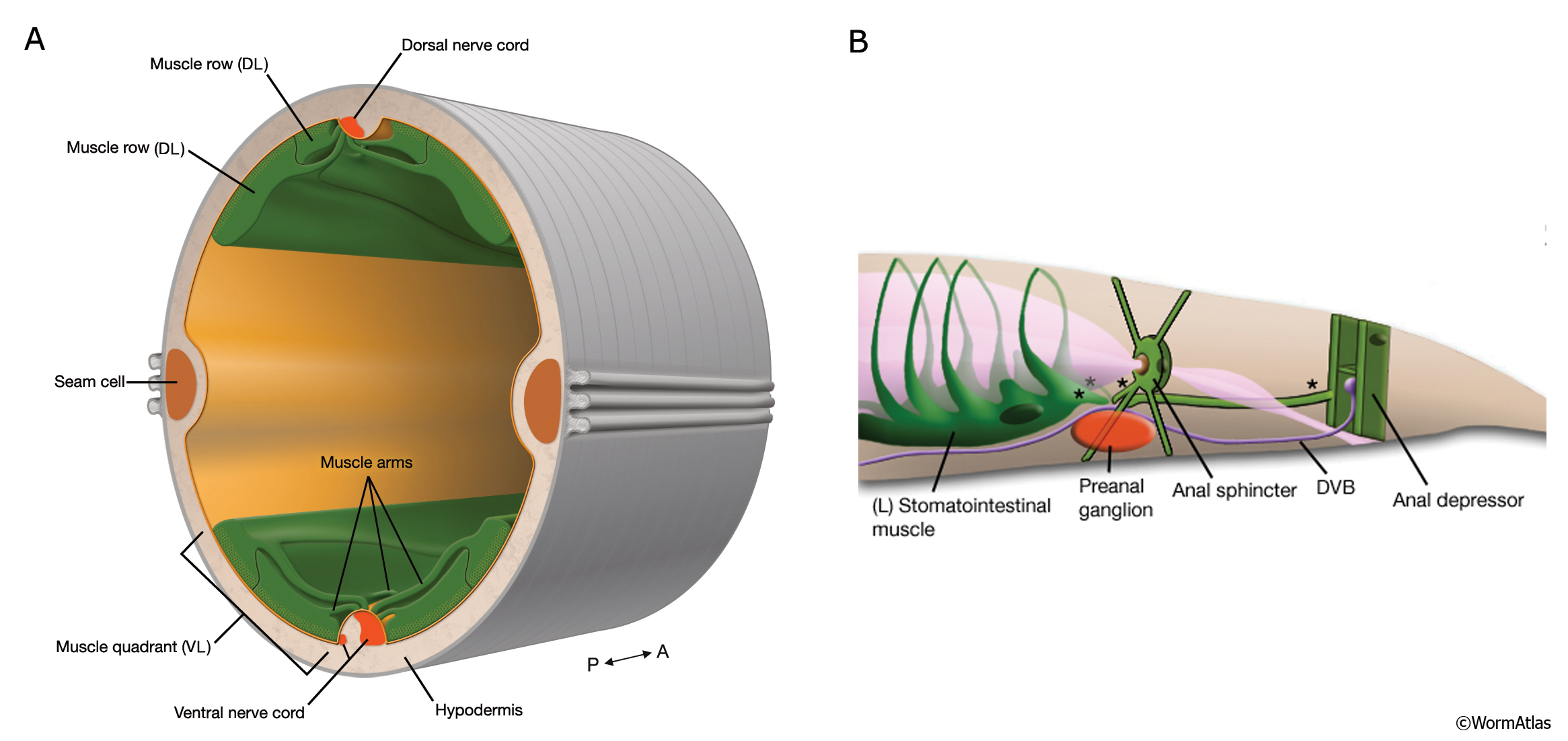
🔬 Model organism advantages
C. elegans is:
- Transparent
- Fast-growing (3–5 days to adult)
- Fully mapped cell lineage
- Genetically tractable
- ~83% of its proteome has human homologs
🧬 Immune relevance
- Lacks adaptive immunity
- Relies entirely on innate immunity → Similar to hibernating bears
🧪 Feeding feasibility
Although worms do not naturally consume blood:
- They can digest human red blood cells
- They survive on serum albumin nanoparticles → Supports feasibility of bear serum exposure
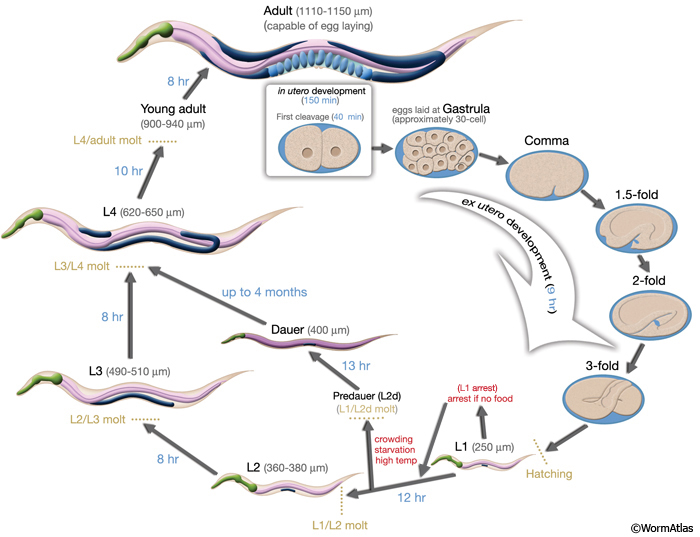
💤 The Dauer State: Worm Hibernation
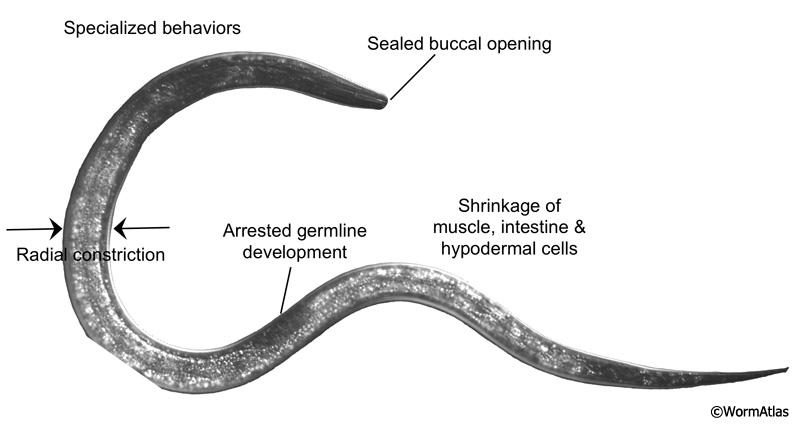
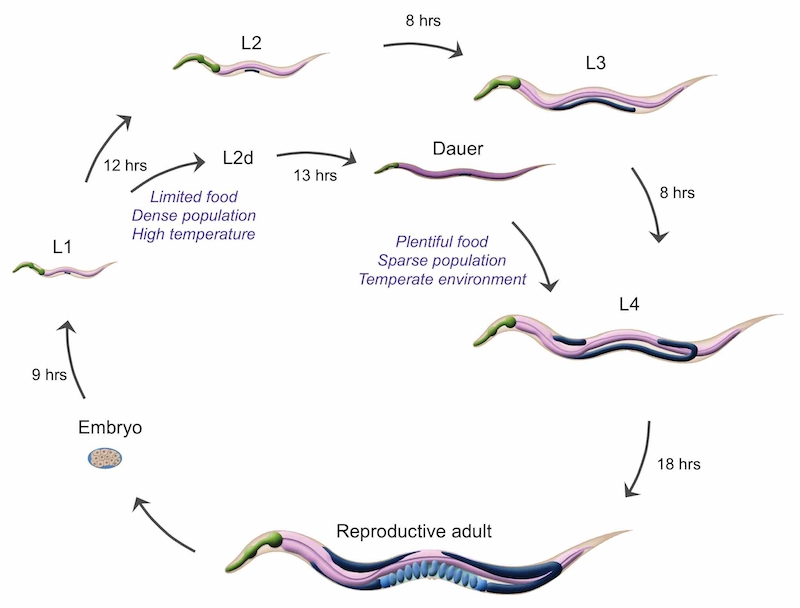
🧊 What is dauer?
A stress-resistant, non-feeding larval state triggered by:
- Low food
- High population density
- Temperature stress
Key features:
- Halted development
- Thickened cuticle
- Sealed mouth
- Reduced movement
- Months-long survival
🔋 Metabolic adaptation
- Fat and carbohydrate storage
- Resistance to oxidative stress
- Upregulation of:
- HSP90
- Superoxide dismutase
If conditions improve, dauers rapidly resume normal development.
🔗 Dauer Signaling Pathways (Highly Conserved!)
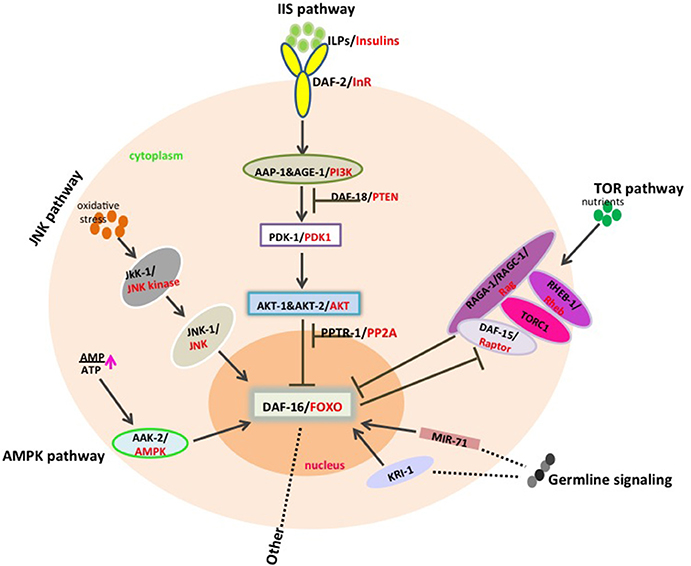

Dauer formation is controlled by pathways also central to human biology:
- Insulin / IGF-1
- TGF-β
- TOR (mTOR)
- Steroid hormone signaling
- Sensory input
🧬 daf genes
- Daf-d: cannot form dauer
- Daf-c: form dauer even under good conditions
🍬 Insulin-Like Signaling in C. elegans
🧠 Core components
- Single insulin/IGF receptor: DAF-2
- Downstream cascade:
- AGE-1 (PI3K)
- PDK-1
- AKT-1 / AKT-2
- Central transcription factor: DAF-16 (FOXO)
⚖️ Functional switch
- High insulin signaling → DAF-16 inhibited → growth
- Low insulin signaling → DAF-16 enters nucleus →
- Stress resistance
- Longevity
- Dauer programs
Reducing DAF-2 signaling can double lifespan.
💪 Muscle Structure & Atrophy
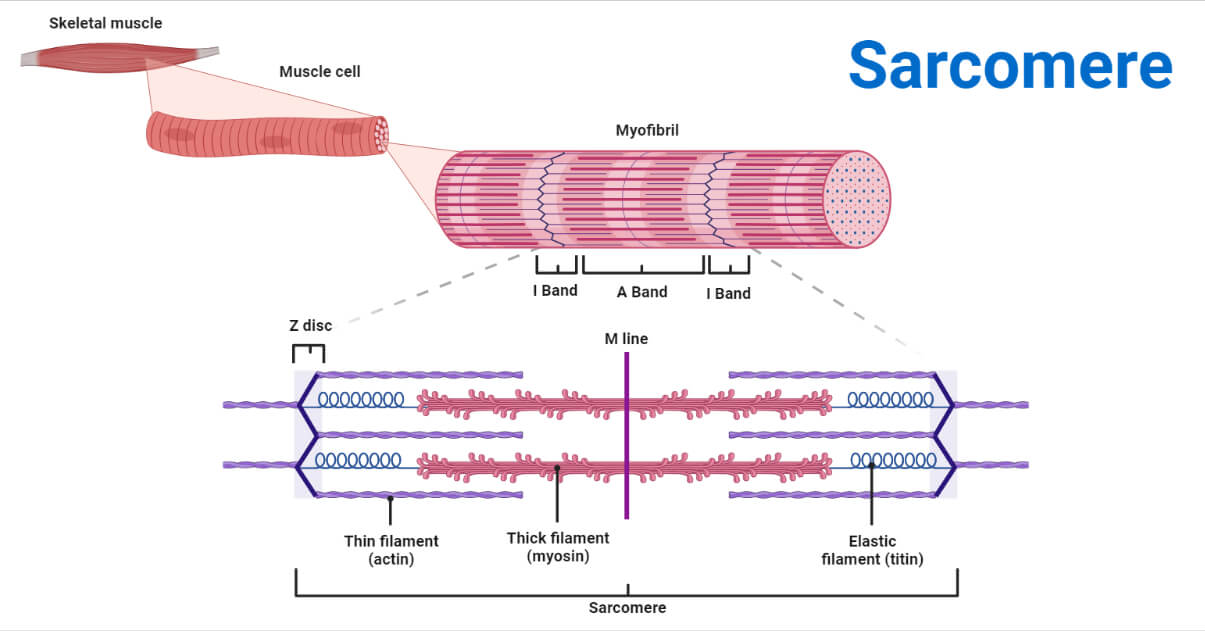


🧬 Skeletal muscle basics
- Muscle fibers (myofibers) contain:
- Sarcomeres
- Actin (thin) & myosin (thick) filaments
- Contraction depends on:
- Ca²⁺ release
- Troponin–tropomyosin shift
- ATP-driven cross-bridge cycling
🪱 Worm muscle vs human muscle
- Body wall muscle is functionally similar
- Differences:
- No satellite cells
- No regeneration
- 95 mononuclear muscle cells
- Sarcomere organization is conserved
📉 Mechanisms of Muscle Atrophy
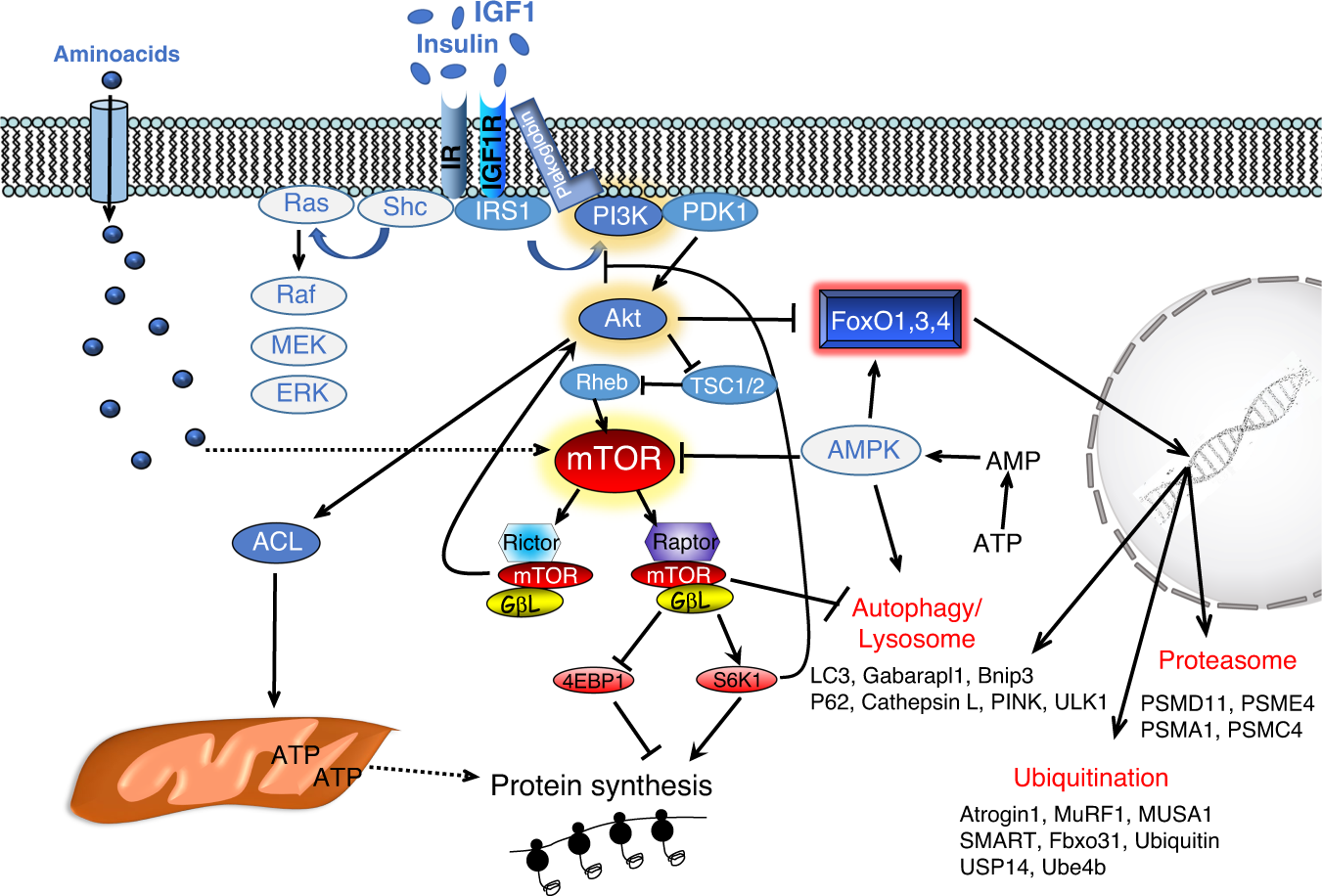


Muscle homeostasis = balance between:
- Protein synthesis (mTOR, AKT, SGK1)
- Protein degradation:
- Ubiquitin–proteasome system
- Autophagy
- Calpains
🧠 FoxO as a master regulator
- Activates catabolic genes
- Suppressed by AKT/SGK1
🧬 Evidence from C. elegans
- Starvation induces muscle atrophy
- clp-4 (calpain) mutants:
- Lose only ~21% muscle size
- vs ~44% in wild type → Calpains actively promote muscle degradation
🎯 Big Picture Takeaway
This project integrates:
- 🐻 Bear hibernation biology
- 🧪 Blood-borne protective factors
- 🪱 C. elegans as a scalable in vivo model
- 💪 Muscle and mitochondrial health
The ultimate goal is to uncover circulating, evolutionarily conserved mechanisms that protect muscle during extreme inactivity—knowledge with major implications for aging, disease, and space biology 🚀